Latest Articles
EnviroTech Europe 2022 Brochure now available

Advanced solutions for vapour degreasing, corrosion protection, metal cleaning and surface treatments.
EnviroTech Europe are specialists in metal, plastic and other substrate cleaning, pre-treatment and protection. We offer a range of approved products tailored to your industry, which are cost effective, energy efficient, safe for operators and the environment.
We are pleased to introduce our range of products in a new 2022 products brochure, viewable on our website and downloadable in PDF format.
Our products are used in the following industries:
Electronics and Electrical
Environmental
General Cleaning
Industrial and Manufacturing
Marine and Maritime
Mechanical Engineering
Medical Device Manufacturing
Military
Motorsport
Power Generation
Railways and Automotive
To view our 2020 Product Brochure please visit www.envirotech-europe.com/brochure.
All products are supplied and supported by EnviroTech Europe Ltd.
Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
Vapour degreasing solvent for precision metal cleaning

EnSolv® CC-A Vapour degreasing solvent for precision metal cleaning
The EnSolv® CC-A formulation for precision metal cleaning responds to the need for high performance vapour degreasing solvents for critical cleaning in general engineering, aerospace, medical optical and electrical industries.
EnSolv® CC-A removes cutting oils, lubricants, lapping compounds grinding pastes and water based cutting oils or fluids. This complex synergistic blend utilises environmentally compliant solvents combined with selected surfactants to remove oils and lubricants to the highest standards for critical cleaning applications and releases and suspends inorganic solids and soils such as fingerprints, polishing compounds and grinding paste from finely finished and highly polished surfaces such as stainless steel and plated parts, optical lenses and components for medical appliances.
EnSolv® CC-A is a proprietary blend of inhibitors and stabilisers for the solvents to prevent water staining and corrosion of yellow metals such as copper, brass and bronze which can be a problem with some other solvents.
EnSolv® CC-A is non-flammable, stable and specifically formulated for use in any vapour degreasing equipment with the added advantage of lower solvent usage, greater stability and reduced costs. The formulation is compliant with all current environmental and user safety legislation.
Product Benefits
Designed for vapour degreasing
Removes oil, grease and soils fast
Simple drop-in replacement for n-propyl bromide and trichloroethylene
Low surface tension
Reduced costs
Compatible with all metals
User friendly
Increased production
Faster cleaning cycles
Components easily handled after cleaning
Safe for the environment
Talk to us about whether EnSolv® CC-A is the best choice for your application. Advice, literature, changeover instructions are all available to make the change quickly, easily and at no extra cost of usage.
FURTHER INFORMATION
Please visit our website https://www.envirotech-europe.com/ensolvcc-a for information about other uses and applications.
Visit www.envirotech-europe.com/applications-and-case-studies for information about uses and applications for all EnviroTech Europe products.
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
Related Posts
Non chlorinated industrial solvent cleaners
Clarea® industrial solvent cleaners – for safe removal of dirt, oil, grease and other contaminants

Clarea® industrial solvent cleaners are a range of highly refined hydrocarbon based
degreasing solvents with very high cleaning power and low odour, which can be used as an
alternative to other hazardous solvents.
Used throughout industry to remove oils, greases, hydraulic fluids, cutting fluids and a wide
variety of hydrocarbon based protective coatings. The solvent cleaners can be used in dip,
brush or in hand-wipe applications.
Clarea® products leave minimal residues on drying surfaces and are compatible with all
metals and many plastics and composite materials. Contains no halogens such as chlorine or
fluorine.
Clarea® HC40 has a relatively fast evaporation rate with low odour and is suited to hand
wipe and brush cleaning and air dries. Flash Point over 40°C.
Clarea® HC62 is a non-carcinogenic solvent mixture that is safe to use in many applications
and is a not classified as flammable. Flash Point over 60°C.
Clarea® industrial solvent cleaning solvents are one of the ranges of metal cleaning and
surface treatment products from EnviroTech Europe Ltd.
EFFICIENT AND ECONOMICAL
- Versatile and cost-effective solutions for all your industrial cleaning
- Minimal odour and low toxicity for operators
- Multipurpose uses can reduce the inventory of cleaning fluids.
- Flexible uses – evaporation rates suitable for hand wipe, spray, or immersion tank cleaning
- Clarea HC40 for brush and hand wiping, air dries. Flash point 40°C
- Clarea HC62 for spray or immersion cleaning Flash point 62°C
- Contains no chlorine, other halogens or stabilisers
- Compatible with all metals and composites and most plastics
- Excellent pre-cleaner for paint or powder coatings
- Safe, reliable, environmentally friendly cleaning
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
Related Posts
Latest News
SuperCORR A Keeps Flight MH370 search planes flying
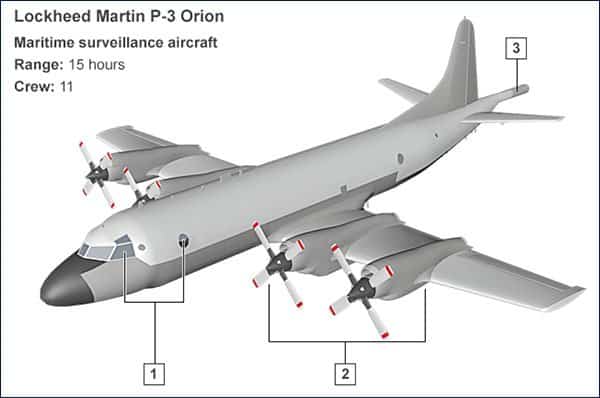
ABOVE:
1. The Lockheed Martin P-3 Orion is equipped with radar and infrared sensors as well as observation posts to help detect any debris on the surface of the ocean. It also has three cameras beneath the landing gear capable of zooming in for a closer look.
2. The four-engine turboprop plane is designed to fly low and slow to aid surveillance. Once it has reached the search location, one or two outer engines can be turned off to preserve fuel and extend the surveillance time.
3. The plane is also fitted with a magnetic anomaly detector (MAD) – used for detecting submarines underwater. The aircraft also has acoustic detectors, which are able to detect sound 1,000ft (304.8m) below the surface of the ocean.
SuperCORR A lubricant keeps Flight MH370 search planes flying
SuperCORR A the lubricant and anti-corrosion formulation from the CORR-EX division of EnviroTech Surface Technologies is helping in the search for the missing Malaysia Airlines flight MH370 passenger jet.
Lockheed Martin AP-3C Orion’s aircraft of the Royal Australian, Korean and Japanese Air Forces used for anti-submarine and maritime surveillance are still searching for debris in a vast area of ocean, bigger than the land area of Europe, southwest of Perth. The maintenance schedules specify SuperCORR A to lubricate and protect the flap tracks and screw jacks in the wings of the aircraft.
SuperCORR A lubricant and protective coating is widely used by the military, electronics and aerospace industries for critical applications to give the protection needed in extreme conditions. SuperCORR Adeposits a dry protective film with excellent corrosion protection and lubricant properties plus the added advantage of a hydrophobic surface rejecting water that ensures the easiest and best connections for very close spaced connectors, contacts, wiring and moving parts.
The U.S. Navy selected SuperCORR A after exhaustive testing using American Society for Testing and Materials (ASTM) Standard B117- Standard Practice for Operating Salt Spray Apparatus. SuperCORR A, a Type I, Grade B Corrosion Preventative lubricant out-performed 11 other products in comparative testing to identify a better product to protect and lubricate the flap tracks and screw jacks on the aircraft.
The flap tracks, located in the aircrafts wings, are what the flaps slide on when they move up or down to lower or increase speed. The screw jacks engage and retract the wing flaps. Corrosion on any of the surfaces can lead to snatching or vibration which can affect the pilots control.
Due to constant operation in salty and corrosive atmospheres which need post-flight rinses and monthly washing down of the aircraft re-lubrication and corrosion treatment for the flap tracks and screw jacks is required each time, with conventional lubricants, to prevent rusting. SuperCORR A was evaluated against competitive products under the Federal Test Method Standard #791B, using a five percent Salt Spray Corrosion Test.
SuperCORR A far exceeded the other products evaluated. After over 200 hours of continuous exposure to salt spray corrosion, SuperCORR A’s protection actually increased over time where the other similar products failed early or contributed to an increase in corrosion.
The accelerated salt fog corrosion testing demonstrated that the application of SuperCORR A which complies with MIL-L-87177A also increased electrical operation to 1400 hours versus 100 hours for the control product used at that time. Technical manuals were updated to include the application for electrical and mechanical parts for civilian and military operations.
The most important conclusion from the historical data and prototype testing is the availability of this excellent corrosion preventive compound that has dry film lubricant properties. The application of SuperCORR A on aircraft components can reduce maintenance man hours, reduce part replacement costs, increase life of aircraft, increase safety, and increase readiness.
Estimates for the maintenance cost for the US Air Force F-16 fleet can reach $500 million per year, the use at all military branches could reach billions of dollars per year. Applications at locations tested by the U.S. Air Force are not normally treated with corrosion prevention and control lubricants (CPC’s) These are the electrical connectors that are susceptible to subtle and not so subtle forms of corrosion that could interfere with the electrical operation of the F-16.
Testing by the U.S. Navy at NADEP Jacksonville incorporates not only electrical connectors, but mechanical and structural components as well. Future uses will also include ground support equipment. The properties of SuperCORR A are such that it can be used on a wide variety of applications and any materials, metal or plastic. Properties of the SuperCORR A far exceed the requirements defined by the MIL-L-87177A specification. Many beneficial properties of the product are not required in the MIL specification.
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
EnSolv and the Environment – Update from the Co Chairman of the United Nations Ozone Assessment panel on nPB.
CONTENT ON THIS PAGE IS RETAINED FOR INFORMATION ONLY
DUE TO n-Propyl bromide now being included in Annex 14 of REACH
EnviroTech Europe Ltd (ETE) continue to publish it as historical information and to record changes occurring in legislation which have affected decisions on formulations and equipment used in vapour degreasing - the most effective, quickest, flexible and cheapest cleaning system used in industry.
EnSolv® invented and patented by EnviroTech has been a market leader all over the world for vapour degreasing. It is based on n-bromopropane (nPB) which now cannot be used as a vapour degreaser within the UK or EU without authorisation.
Archived information about EnSolv® can be found using the Discontinued Products & Resources navigation menu on this page.
Using experience accumulated over 40 years supplying and supporting users of the vapour degreasing process ETE specialists have developed “drop in” alternatives:
ProSolv®, ProSolv5408e® and EnSolv CC-A® give the same or improved level of performance and economy as the original EnSolv® products.
Please contact our advisers who are available to discuss your needs and propose the best replacement product.
Or please click here to return to our vapour degreasing products homepage for information about our current products.
 |
| view/ download this document in Adobe Acrobat Format |
Analysis of Status on Science of n-PB Effects on Ozone
White Paper By Don Wuebbles (Department of Atmospheric Sciences – University of Illinois).
November 11, 2014
There is disagreement between the use of scientific information by the European Union and the United Nations Environmental Panel / World Health Organisation (UNEP/WHO) the authority for ozone depletion assessment. Dr. Don Weubbles acknowledged as one of the world authorities on ozone depletion and who Co Chaired the UNEP/WHO 2014 ozone assessment panel explains in detail why the official world study disagrees with the European Union Environment Agency 2014 assessment as not being supported by science.
EnSolv is safe for the environment backed by the most sophisticated and knowledgeable world scientists.
Analysis of Status on Science of n-PB Effects on Ozone
Don Wuebbles
Department of Atmospheric Sciences
University of Illinois
Urbana, IL
Phone (217) 244-1568
Email:Wuebbles@illinois.edu
November 11, 2014
I co-led chapter 5 (with Neil Harris of Cambridge University) of the 2014 WMO-UNEP ozone assessment that is currently in press (the summary, Assessment for Decision Makers, was released in September 2014 (WMO-UNEP, 2014)). Chapter 5, titled Scenarios and Information for Policymakers, of the full assessment, examines the latest understanding of a number of Ozone Depleting Substances (ODSs) including the available analyses of Very Short Lived Substances (VSLS) like n-propyl bromide (n-PB; C3H7Br). The full assessment will be released in early 2015 as a web-based report.
In Chapter 5 of the full assessment, we include the following text related to the evaluation of VSLS like n-PB:
Table 5-3 shows analyses of the spatial dependence in ODPs for VSLS primarily based on results using different versions of the NCAR global 3-D model (Wuebbles et al., 2009, 2011; Patten and Wuebbles, 2010; Youn et al., 2010; Patten et al., 2011). Note that this model calculates an atmospheric lifetime of 53.7 years for CFC-11, so the published ODPs would not be significantly affected by the revised SPARC (2013) lifetime for CFC-11. In these studies, the VSLS examined all have quite small ODPs based on emissions occurring primarily at midlatitudes. New approaches for estimating VSLS ODPs have been developed since WMO (2011) based on Lagrangian models (Tegtmeier et al., 2012; Pisso et al., 2010; Brioude et al., 2010), with similar findings to previous studies, except for emissions in the tropics where a different treatment of convection may allow for more VSLS (and their products) to reach the stratosphere.
The reported atmospheric lifetime and Ozone Depletion Potential (ODP) for n-PB in Table 5-3 for midlatitude emissions (30-60N), based on the 3-D chemistry-climate modeling studies of Wuebbles et al. (2011), are 24.7 days and 0.0049, respectively.
At the same time, we noted:
Earlier studies (Wuebbles et al., 1999, 2001; Olsen et al., 2000; Bridgeman et al., 2000) have shown that the ODPs for short-lived compounds depend greatly on when or where the emissions occur, with the largest ODPs being found for emissions in the tropics. Although it is generally expected that most emissions from anthropogenic emissions of VSLS will occur at Northern midlatitudes, there is no guarantee of this and the locations of future emissions could change.
In addition, we also reported upon an indirect study of the ODP using a semi-empirical approach based on the Lagrangian model analysis of Brioude et al. (2010). The results in Table 5-4 of WMO-UNEP Chapter 5 are taken from the Supplementary materials for that published paper. The results in Table 5-4 are quite a bit higher than Wuebbles et al. (2011) and show an ODP for n-PB of 0.0235 (0.0150-0.032) for North America emissions and 0.0150 (0.0070-0.0260) for European emissions. However, as noted in the chapter, the Brioude et al. results may be an overestimate because they do not properly account for reaction loss in the troposphere and therefore may have overestimated the amount of n-PB reaching the stratosphere. As stated in the Chapter:
The recent modeling studies also re-emphasize the point that VSLS ODPs are very dependent on the location of emissions, and not just the latitude; for example, by co-location with efficient vertical transport by deep convection into the stratosphere (semi-empirical ODPs as a function of specific locations of emissions based on Brioude et al. (2010) are shown in Table 5-4). Brioude et al. (2010) showed that these factors are more important than regional variations in VSLS losses by OH or photolysis. Using CO-like emissions to represent anthropogenic VSLS, they estimated ODPs for various compounds and found maximum ODPs over the Indian sub-continent varying from 0.079 in winter to 0.29 in summer for n-propyl bromide (C3H7Br or nPB) and from 0.13 in winter to 0.83 in summer for CH3I. Pisso et al. (2010) applied their new methodology to an nPB-like tracer with a lifetime of 20 days. They also found higher ODPs over southeast Asia in the summer (and over western Pacific in winter). In July in the tropics (30°N-30°S), ODPs varied from 0.33 in runs with convection to 0.17 in runs with no convection. Locally, values over southeast Asia are as high as 1.00. In general the results from these Lagrangian studies predict higher ODPs regionally compared to the global model results. These differences highlight uncertainties in simulating the transport of VSLS, with boundary layer mixing, convection depth and advection strength all possibly leading to local differences in VSLS delivery to the stratosphere (e.g., see Hossaini et al., 2012b; Feng et al., 2011; Hoyle et al. 2011). The global model studies (e.g., Wuebbles et al., 2011) used a full chemical treatment for VSLS and CFC-11 degradation in the stratosphere and more realistic degradation and wet deposition schemes for VSLS in the troposphere than the Lagrangian based studies (e.g., Tegtmeier et al., 2012; Pisso et al., 2010), leading to less VSLS reaching the stratosphere. Overall, these results point to potentially more important impacts from VSLS if emissions occur in regions close to convective regions in the tropics.
The 2014 report from the European Environment Agency, Ozone-Depleting Substances 2013, examines the European reporting requirement for n-PB and determines a total import of nPB to Europe of 1014.3 metric tonnes and a weighted ODP-tonnes for imported nPB of 101.4. This suggests they used an ODP of 0.1 for n-PB. This is far too large by either the Wuebbles et al. (2011) or the Brioude et al. (2010) analyses by roughly a factor of 6.7 for Europe emissions using the Brioude et al approach and a factor of 20.4 using the Wuebbles et al. 3-D model results. Because Wuebbles et al. may have underestimated the convection based on the observations used in the Brioude et al. study, the reality is likely somewhere between the two results, but this still suggests that the European Environment agency still used an ODP a factor of 10 or more too large. The science does not support the ODP used by the European Environment Agency.
References
Bridgeman, C.H., J.A. Pyle, and D E. Shallcross, A three-dimensional model calculation of the ozone depletion potential of 1-bromopropane (1-C3H7Br), J. Geophys. Res., 105, 26,493-26,502, 2000.
Brioude, J., R.W. Portmann, J.S. Daniel, O.R. Cooper, G.J. Frost, K.H. Rosenlof, C. Granier, A.R. Ravishankara, S.A. Montzka, and A. Stohl, Variations in ozone depletion potentials of very short-lived substances with season and emission region, Geophys. Res. Lett., 37, L19804, doi: 10.1029/2010GL044856, 2010.
European Environment Agency, 2014: Ozone-Depleting Substances 2013. EEA Technical Report No. 14/2014, Copenhagen.
Olsen, S.C., B.J. Hannegan, X. Zhu, and M.J. Prather, Evaluating ozone depletion from very short-lived halocarbons, Geophys. Res. Lett., 27, 1475-1478, 2000.
Patten, K.O., and D.J. Wuebbles, Atmospheric lifetimes and Ozone Depletion Potentials of trans-1-chloro-3,3,3-trifluoropropylene and trans-1,2-dichloroethylene in a three-dimensional model, Atmos. Chem. Phys., 10, 10867-10874, 2010.
Patten, K.O., V.G. Khamaganov, V.L. Orkin, S.L. Baughcum, and D.J. Wuebbles, OH reaction rate constant, IR absorption spectrum, ozone depletion potentials and global warming potentials of 2-bromo-3,3,3-trifluoropropene, J. Geophys. Res., 116, D24307, doi:10.1029/2011JD016518, 2011.
Pisso, I., P.H. Haynes, and K.S. Law, Emission location dependent ozone depletion potentials for very short-lived halogenated species, Atmos. Chem. Phys., 10, 12025-12036, 2010.
SPARC (Stratospheric Processes And their Role in Climate), Report on the lifetimes of stratospheric ozone-depleting substances, their replacements, and related species, M. Ko, P. Newman, S. Reimann, S. Strahan (Eds.), SPARC Report No. 6, WCRP-15, Zurich, Switzerland, 2013
Tegtmeier, S., K. Krüger, B. Quack, E.L. Atlas, I. Pisso, A. Stohl, and X. Yang, Emission and transport of bromocarbons: from the West Pacific ocean into the stratosphere, Atmos. Chem. Phys., 12, 10633-10648, doi:10.5194/acp-12-10633-2012, 2012.
WMO-UNEP (coauthor), 2014: Assessment for Decision-Makers: Scientific Assessment of Ozone Depletion 2014. WMO Global Ozone Research and Monitoring Project – Report No. 56, Geneva, Switzerland; also available on WMO website.
Wuebbles, D.J., K.O. Patten, M.T. Johnson, and R. Kotamarthi, New methodology for Ozone Depletion Potentials of short-lived compounds: n-Propyl bromide as an example, J. Geophys. Res., 106, 14551-14771, 2001.
Wuebbles, D.J., D. Youn, K. Patten, D. Wang, and M. Martinez-Aviles, Metrics for ozone and climate: Three-dimensional modeling studies of Ozone Depletion Potentials and Indirect Global Warming Potentials, in Twenty Years of Ozone Depletion, C. Zerefos, G. Contopoulos, and G. Skalkeas, editors, Springer Publishing, Dordrecht, The Netherlands, doi: 10.1007/978-90-481-2469-5, p. 297-326, 2009.
Wuebbles, D.J., K. Patten, D. Wang, D. Youn, M. Martínez-Avilés, and J. Francisco, Three-dimensional model evaluation of the Ozone Depletion Potentials for n-propyl bromide, trichloroethylene and perchloroethylene, Atmos. Chem. Phys., 2011, 11, 2371-2380, 2011.
Youn, D., K.O. Patten, D.J. Wuebbles, H. Lee, and C.-W. So, Potential impacts of iodinated replacement compounds CF3I and CH3I on atmospheric ozone: a three-dimensional modeling study, Atmos. Chem. Phys., 10, 10129-10144, 2010.
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
Oxygen Cleaning
Cleaning For Oxygen Service Whitepaper
White Paper By Phil Dale (Co-contributor Handbook for Critical Cleaning – Liquid Displacement Drying Techniques).
Cleaning for oxygen service is best defined as the removal of combustible contaminants from the surface of any equipment or system in oxygen service, including all parts thereof. Essentially, any component that may come into contact with an oxygen rich environment.
The combustible contaminants include organic and inorganic substances such as hydrocarbon material for example oils and greases, paper, fibre, coal dust, solvents, weld slag, rust, sand and dirt. If these contaminants are not removed properly, in a worst case scenario, this can cause combustion in an oxygen atmosphere or at the least rejection of the product due to unacceptable product purity.
Oxygen in its own right is not flammable but it supports combustion. Oxygen can react with most materials. The higher the oxygen content and/or pressure in a system the more vigorous the combustion and the lower the ignition temperature required. Materials that do not normally ignite in atmospheric air will burn and may explode in an oxygen rich environment. In addition the oxygen rich environment will give rise to a higher flame temperature and combustion velocity and the devastating consequences thereof.
The recognition of oxygen’s reactivity has led to stringent requirements regarding the cleanliness of equipment in oxygen service. Strict guidelines exist to ensure that care must be taken in the selection of equipment including all materials and components, all of which need to be oxygen compatible. They must also be free from combustible contaminants as described above.
With this in mind special consideration must be given to any cleaning processes employed in the manufacture and maintenance of all oxygen service systems.
Specific consideration must be given to the following:
- cleaning standard to be achieved (how clean is clean?)
- cleaning procedure specified (or not)
- cleaning agent to be used
- surface properties of the parts to be cleaned
- shape and geometry of the material
- types and amounts of contaminants
- the degree of automation required
The size and capacity of the equipment is determined from:
- the size of the material or components to be cleaned
- the required throughput
Your starting point should be the cleaning standard and procedure. For example *G93 indicates that solvent cleaning is preferable. Solvent cleaning and solvent vapour phase cleaning of components consists of the removal of contaminants by immersion in the solvent, possibly with the addition of ultrasonic agitation and the action of continued condensation of solvent vapour on the component surfaces. The procedure requires that the oxygen equipment, system or component is colder than the solvent boiling point. This allows the vapour to condense on the components and perform a final rinse.
The major significant advantage of solvent cleaning is that re-vaporised solvent is always clean and the contaminants remain in the evaporator liquid section which requires only periodic cleaning out, thus causing a reduction in the frequency of system downtime.
It is also important to note **G127–95 (Reapproved 2000). The effectiveness of a particular cleaning agent depends upon the method by which it is used, the nature and type of the contaminants and the characteristics of the article being cleaned, such as size, shape, and material. Final evaluation of the cleaning agent should include testing of actual products and production processes.
All equipment must, together with the cleaning chemistry, fulfil as a minimum the legislation for health, safety and environment.
The choice of equipment has to be based on the efficiency of cleaning versus cost bearing in mind what is the cost of the problem? If there is no cost there is no problem.
The efficiency is controlled by utilising typical samples, written procedures and requested criteria for cleanliness.
If you need to clean to ASTM G93 – 03(2011) Standard Practice for Cleaning Methods and Cleanliness Levels for Material and Equipment Used in Oxygen-Enriched Environments then all of the above needs to be given due consideration.
*G93 – Standard Practice for Cleaning Methods and Cleanliness Levels for Material and Equipment Used in Oxygen-Enriched Environments
**Designation: G127 –95(Reapproved 2000) Standard Guide for the Selection of Cleaning Agents for Oxygen Systems.
Handbook for Critical Cleaning, Second Edition – 2 Volume Set Hardcover – April 4, 2011by Barbara Kanegsberg (Editor), Edward Kanegsberg (Editor)
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
New study disputes TLV for nPB
A detailed comprehensive review of 1-bromopropane studies confirm current recommendations by EnviroTech for occupational exposure levels for safe usage of EnSolv® as a vapour degreasing solvent.
 |
| view/ download this document in Adobe Acrobat Format |
Update on a safe occupational exposure level for 1-Bromopropane
Prepared for EnviroTech Europe, Ltd.
Prepared by Dr. Mark Stelljes
SLR International Corporation
September 2014
Executive Summary
This paper re-evaluates EnviroTech Europe’s (ETE’s) current occupational exposure level recommendation of 100 ppm for 1-bromopropane (1-BP) [106-94-5] in the vapor degreasing industry in light of the recent lowering of the ACGIH Toxicity Threshold Value (TLV) from 10 ppm to 0.1 ppm. The 0.1 ppm value is based on a study of 86 workers exposed to 1-BP during its manufacturing in China in four different facilities. The authors reported significant effects at all 1-BP exposure levels down to 1.28 ppm. The 1-BP in these facilities had concentrations of the isomer 2-bromopropane [75-26-3] (2-BP) present as a contaminant at about 10-20 times the level sold for vapor degreasing.
There are several factors that undermine the conclusions reached in the paper that a concentration of 1.28 ppm resulted in toxicity in exposed workers. These factors were related to:
(1) Exposure measurements – passive rather than active samplers were used, and concentrations varied by more than tenfold for the same activity.
(2) Exposure via other routes in addition to inhalation – described worker activities indicate substantial dermal exposure, which increases the overall dose of the chemical relative to just inhalation exposure.
(3) Exposure to other chemicals – at least 20% of the workers were previously exposed to 2-bromopropane
(2-BP), and no testing was done for other chemical exposure.
(4) Statistical methods and interpretations – instead of using paired patient-exposure data, authors categorized exposure into groups (e.g., high, low); this resulted in apparent statistical relationships that may not be biologically relevant.
(5) Lack of robust dose-response relationships – when evaluating typical doseresponse relationships, only a single parameter (vibration sense in the toes, a subjective parameter) was shown to be significantly different across all dose levels.
(6) The outcome of the subjective vibration sense test was in part dependent on the testing doctor – this dependency should remove the test and its results from consideration in the paper as a scientifically defensible endpoint.
When all of this information is considered as a whole, it is unlikely that the 1.28 ppm lowest effect concentration reported in the paper is accurate. The interpretations in the Li et al. study are inconsistent with expectations based on the ways in which 1-BP acts in rodents relative to humans. Studies on how 1-BP acts in the body of rats and mice and studies on metabolism of the chemical in humans indicate that humans should be no
more sensitive to 1-BP than either of these rodents.
Based on the weight of evidence available for the toxicity of 1-BP in humans and rodents, there is no credible scientific reason to target an occupational concentration as low as 10 ppm or 0.1 ppm. ETE’s current recommendation of 100 ppm should be maintained, and employers together with vapor degreasing personnel should not be concerned about the much lower levels recommended by the ACGIH.
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
ACGIH Threshold For N-Propyl Bromide Solvents
What You Should Know About The ACGIH’s Threshold For nPB Solvents
If you work with industrial chemicals, materials or solvents then you may have heard of the American Conference of Governmental Industrial Hygienists (ACGIH). The ACGIH is a scientific, but non-governmental body of industrial hygienists that publishes Threshold Limit Value (TLV) opinions on chemicals such as n-propyl bromide the base solvent for EnSolv formulations. These TLV opinions are not held to the same standards as limits set by an organization such as OSHA in the USA, but are still published in books available on the ACGIH website. You or someone else in your company might be familiar with these TLVs.
In March 2014, the ACGIH published a new TLV for n-propyl bromide (1-bromopropane). This TLV was set at 0.1ppm, and refers to “commercial grade bromopropane (99% 1-BP with 0.1%-0.2% 2-bromopropane).” 2-bromopropane is also known as isopropyl bromide (iPB). Modern nPB manufacturing processes result in nPB with <0.01% iPB contamination, which is of a pharmaceutical quality.
The 1-BP (nPB) properties referenced by the ACGIH rely to some degree on information first published in 1999 and 2000, which contains information that is out of date and inaccurate when referring to today’s purity of 1-BP. Some of the things to keep in mind regarding ACGIH TLV opinions include:
- TLVs are not determined by speaking openly with scientific experts, but in closed-
- Door meetings.
- There is no scientific consensus backing up ACGIH’s findings.
- ACGIH TLV opinions may involve conflicts of interest and uncertain science.
- The ACGIH is a not-for-profit organization that funds its activities by selling books; it is not a government-sanctioned panel of experts.
The IBSA et al. v. ACGIH lawsuit documents a number of these concerns involving the ACGIH and how they determine their TLV opinions. There is nothing worse than misinformation in our field – not only are safer chemicals such as nPB made to seem dangerous, but chemicals that are unsafe or unsuitable might be seen as acceptable alternatives.
It is of great concern to that in spite of the very demanding regulations already in effect in Europe some manufacturers of very expensive “new” fluorocarbon alternatives to the less expensive conventional solvents choose to imply that recent ACGIH recommendation for a reduction of exposure limits will have legal effect in Europe. This wrong and no credence should be given by users to any “recommendations” except those issued by government regulators.
The EU will establish DNEL (Derived No Effect Level) recommendations under the REACH legislation in due course and until these are issued all properly designed and maintained equipment is perfectly safe for use for vapour degreasing and any suggestion to the contrary may cause unnecessary confusion and restrict the use of this very useful technology.
EnviroTech Europe urges you and others in your company not to rely solely on TLVs, but rather on official and informed sources instead (See US EPA SNAP Approval for 1-BP). For more information on the properties of nPB and related solvents, please visit www.vapour-degreasing.com.
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
Condensation cleaning, the future for solvent degreasing

Innovation driven by legislation
Vapour degreasing is the simplest but most effective degreasing and cleaning process. It has, until recently, been subject to little change since it was first invented in the early part of the last century. The name for the process is a misnomer as the cleaning is actually achieved by solvent vapour condensing on the cooler target parts and the hot liquid solvent dissolving oil and removing dirt.
Vapour degreasing is a mature technology on which legislation is now effecting changes so fundamental that the more accurate name for the process “Condensation Cleaning” should be used to reflect the way in which it works. More importantly the alternatives in equipment design and fundamental differences in the technologies which guarantee its continuing use in the future need to be considered and understood.
In Europe the United States and in other advanced industrialised economies increasingly stringent legislation to control emissions of VOCs and especially solvents has led to new formulations for paints and other coatings where water replaces most of the solvent. In the early days of these changes the quality of the coatings was not as good as solvent based coatings but innovation has driven the development of new polymers and the results are now as good as, if not better than, the solvent based originals.
As with coatings, cleaning systems have had to change. With increased legislation concerning the regulation of health and environmental safety uses of solvent have been targeted and alternatives are encouraged by the relevant authorities and law makers often without serious consideration of unintended consequences.
For example water based cleaning is the obvious alternative but this has many disadvantages compared to condensation cleaning as although the machines are simple and relatively cheap the processes require multiple tanks for immersion or spray processing or long programmed cycles in batch machines for cleaning, rinsing and drying. Water based processes are slow, energy intensive and occupy more floor space than condensation cleaning equivalent where only one tank with small footprint is needed delivering shorter process times and most importantly using minimal energy.
The ideal cleaning process would be Condensation cleaning with water but due to the physical characteristics of the vapour/steam and the inability to carry onto the surface of the targeted parts surfactants to remove the oil, grease and soils and the difficulty of rinsing and especially drying this will only ever be a dream.
Halogenated hydrocarbons are the solvents of choice for Condensation cleaning. The process is essentially simple. A tank with a sump to contain the solvent, heaters at the base and condensation coils around the top section to control the height of the vapour is all that is needed. When heated in the sump halogenated solvents produce, in most cases, a saturated vapour between 3 and 4 times heavier than air at a temperature greater than the ambient temperature of the parts to be cleaned. This allows the solvent vapour to condense on the surface. The condensate dissolves the contaminants such as oil, greases and soils returning the used solvent into the sump of the machine for recycling into vapour which continuously condenses onto the parts until they have achieved vapour temperature when, with no further condensation, the process is complete. Parts removed from the cleaning machines are very clean, warm and dry.
The most common halogenated solvents, in use for commercial purposes, are non-flammable so present no risk in this process. Perchloroethylene, used mainly for dry cleaning, methylene chloride used widely in paint strippers and trichloroethylene used for vapour degreasing were the original materials used based on chlorine chemistry. However continuing concern about the environmental impact and danger to operators by exposure to chlorine based solvents, especially trichloroethylene, has led to a continuing search for safer alternatives.
Genklene from ICI and Chlorothene from Dow Chemicals, both of which were based on another chlorinated solvent 1.1.1. trichloroethane, were to become ubiquitous in the mid part of the last century as a much safer, non carcinogenic replacement for trichloroethylene. An excellent solvent safe for users but which, with increased awareness of the environmental impact of solvents, proved to be depleting the protective ozone layer around the Earth was subsequently banned. Trichloroethylene was then allowed to be used again as a substitute for trichloroethane but with increased restrictions on its use, even though safer materials were already available. With the development of the REACH legislation in Europe, which now classifies trichloroethylene as a human carcinogen, usage will be even more difficult but will probably be authorised in machines which control factory emissions to very low figures close to zero.
Meanwhile n-propyl bromide (nPB) based cleaning solvents such as EnSolv were developed in the United States as a drop in replacement for the ubiquitous 1.1.1.trichloroethane. nPB has an identical profile, stable, non-flammable, with the same physical characteristics such as boiling point and specific gravity and it is an excellent cleaning solvent but without the potential for ozone depletion.
During this time new halogenated blends using trans1.2 dichloroethylene, a highly flammable solvent with similar chemistry to trichloroethylene were also developed for condensation cleaning. The trans 1.2 dichloroethylene is blended with a variety of different fluorocarbon liquids which are not suitable as cleaning solvents, as they have very low solvency, but are used in these blends as a fire retardants. An extremely expensive answer to a simple problem, already solved by the development of machines able to safely use the cheaper solvents.
As with all legislation for chemical use regulation and restrictions drive development of associated processes and equipment. The most important developments in affordable machines to use solvents for condensation cleaning use two different approaches and raise difficult questions as to which is the best most economical and simplest in use.
The single tank hermetically sealed machines favoured by mainly German manufacturers uses a process tank to contain the solvent and a separate storage tank/vapour generator. Baskets with parts to be cleaned are loaded from the top and lowered into the process tank. A lid then closes over the tank and is hermetically sealed. Solvent is pumped into the process tank. Baskets are immersed in the solvent where agitation, ultrasonics or pumped liquid is circulated through the parts.
When the immersion process is complete solvent is pumped to the storage tank and vapour fed to the process tank from a vapour generator for rinsing and drying. Condensing coils/panels controlling the vapour height are linked to coils located within the vapour zone which, when the cycle is complete, collapse the vapour. The process tank is emptied. The saturated air/solvent from the process tank is then recycled through carbon absorption units to remove traces of solvent from the air until the concentration is below 2 grams per cubic metre when the lid opens automatically for the basket of parts, clean and dry, to be removed and replaced with a further basket.
Variations within the hermetically sealed tank are possible using sprays of cold or hot condensed solvent, immersion with or without ultrasonics and revolving baskets to cover most of the problems experienced when processing machined and fabricated parts. Continuous external distillation of the contents of the vapour generator is also an option depending on contamination
The alternative technology is much simpler and cheaper using well tried techniques not requiring the long recovery times of the one tank processes. This is favoured by U.K. based manufacturers. The multilevel system shown in the diagram uses a standard vapour degreasing tank design with a sealed loading section above the process tank.
Work baskets are fed to the load section from a hoist or conveyor. The loading door is then pneumatically sealed to hermetically isolate the process. The sealing lid on the process tank is opened and the basket lowered into the cleaning tank which can be simple condensation cleaning with vapour generator below or an offset generator can be used with the lower section being filled with clean solvent for immersion cleaning with or without ultrasonics or power sprays. Revolving baskets can also be added to improve cleaning in blind or through holes or oil ways in castings and fabrications.
When the process is complete the basket rises to the freeboard area where the condensation coils are located and parts allowed to drain and dry. The tank seal opens to allow the basket into the load section while the lid closes again across the process tank. A fan is then activated which produces a negative vacuum in the load area. The loading door is then partially opened to allow a flow of air through the loading section which is exhausted to atmosphere or can be recycled through carbon absorption systems if no external exhaust is preferred. As baskets entering the loading space contain parts drained and dried only very small amounts of solvent are carried in the exhausted air which is monitored to ensure compliance with local legislation. When this is achieved the door opens fully for removal of basket. The loading section can be fitted with top or side sealed doors allowing baskets to flow through on conveyor systems or be loaded from hoists. No solvent enters the work area.
Both processes will produce parts cleaned to the highest standards but the choice of which offers the best solution needs a little more consideration.
The single tank process is complicated with the need to move liquids in the machine. It is relatively slow as the carbon adsorption process needs to remove high levels of solvent from the recirculated airstream from the process tank. This is inefficient as adsorption rates for carbon fall rapidly with increasing saturation. Final levels of solvent in the tank when the lid opens will be dragged into the workshop and the area in which the operator is working. Long process cycles reduce throughput.
The simpler multilevel systems where movement of liquids is not required are more efficient and offer much faster process times. Cost of manufacture is also cheaper as no liquid movement is involved. The extracted loading section ensures no leakage of solvent into the work area which offers complete safety for operators and factory staff. The disadvantage of the multilevel machine is the height of the equipment compared to the single tank machine. This will often require the equipment be installed in a pit for easy access.
Both designs of process machines fully comply with the emission regulations of the EU and U.S authorities. Which to choose will depend on many factors which should be discussed with both the equipment and solvent suppliers who have the experience to advise on which offers the best process for the application. Equipment and solvent must work together and it is advisable to ensure that the suppliers work closely together to offer a package with high levels of responsible care and product stewardship to ensure the best and safest installation.
The Condensation cleaning process still gives the highest levels of economical cleaning with minimum energy usage, low footprint on the factory floor, safety for the operator, and high production rates with low solvent usage or environmental impact. With the new generation of sealed cleaning machines its future as the process of choice is assured.
Syd Treacher is a consultant for industrial cleaning processes
Drawing by kind permission of CC Hydrosonics Ltd www.cchydrosonics.com
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
Samuel Banners Ltd appointed EnSolv distributors for UK
CONTENT ON THIS PAGE IS RETAINED FOR INFORMATION ONLY
DUE TO n-Propyl bromide now being included in Annex 14 of REACH
EnviroTech Europe Ltd (ETE) continue to publish it as historical information and to record changes occurring in legislation which have affected decisions on formulations and equipment used in vapour degreasing - the most effective, quickest, flexible and cheapest cleaning system used in industry.
EnSolv® invented and patented by EnviroTech has been a market leader all over the world for vapour degreasing. It is based on n-bromopropane (nPB) which now cannot be used as a vapour degreaser within the UK or EU without authorisation.
Archived information about EnSolv® can be found using the Discontinued Products & Resources navigation menu on this page.
Using experience accumulated over 40 years supplying and supporting users of the vapour degreasing process ETE specialists have developed “drop in” alternatives:
ProSolv®, ProSolv5408e® and EnSolv CC-A® give the same or improved level of performance and economy as the original EnSolv® products.
Please contact our advisers who are available to discuss your needs and propose the best replacement product.
Or please click here to return to our vapour degreasing products homepage for information about our current products.

EnSolv is the only realistic safe, economical alternative to the carcinogen trichloroethylene for vapour degreasing.
Responding to the increasingly stringent legislation on solvent usage EnviroTech have over the years reacted to the changes and introduced and developed the solvent minimisation programme which gives help and guidance to users in ways to reduce the amount of solvents used in vapour degreasing to comply with the EU Solvents Emission directive. This is achieved by a combination of working with partners to improve equipment, better solvent handling and more efficient use of the vapour degreasing process. Training staff in proper loading of work baskets, correct timing procedures and many more detailed improvements which when combined not just reduce solvent usage but make it safer for the operator and the environment while considerably reducing costs for users. After discussion with many potential distributors to find a partner with the same ethical standards, passion for customer service and training and with the high level of knowledge to service and support our customers we are very pleased to announce Samuel Banner Ltd will be the distributors for EnSolv in the UK from November 2013.
Established 150 years ago Samuel BANNERS Ltd were granted the patent for White Spirit and from that time they have continued to be involved with solvents manufacturing and distribution. Their extensive storage and distribution depots will offer a fast and efficient delivery for EnSolv throughout the U.K. The respected technical sales team at BANNERS are active in precision engineering, aerospace, medical, electronics and optical industries where critical cleaning is required and have an in-depth knowledge of vapour degreasing to give advice and support to all EnSolv customers. EnviroTech Europe Ltd and Banner Chemicals are both committed to Responsible Care and will continue to advise on legislative or other changes and research for our products to meet the highest quality standards.
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:
A Real Fire Suppression System Problem and Your Electronics and Computer Systems
HFC-227ea is replacing Halon 1301 as the gaseous fire suppression agent for data processing and electronic equipment.
However when activated in a fire situation HFC-227ea will decompose to produce Hydrogen Fluoride. This is a real and ongoing concern. Why? Because Hydrogen Fluoride causes major corrosion problems for electronics and connectors. You may save your equipment but for how long?
In 2004 the Institute of Research and Construction in Canada published a study on HFC-227ea (C3F7H) paper, Thermal decomposition products from fire suppression with HFC-227ea in an electronic facility 1. That study was followed by Corrosion of Electronic Components by Hydrogen Fluoride (HF)2 in 2008 by the Fire Research Program of the National Research Council of Canada. Both studies were published in the Journal of Fire Protection Engineering. These two in depth studies address and prove the very definite concern of Hydrogen Fluoride (HF) corrosion after a gaseous HFC-227ea fire suppression system is used in computer and other electronic environments.
The first published study by the National Research Council of Canada (NRC) in 2004 showed that HFC-227ea, a gaseous fire suppressant, a replacement for Halon 1301, produced at least 5 to 10 times more HF than Halon 1301 under similar fire conditions, however, there was no information available on the amount of thermal decomposition products generated during fire suppression with halocarbon agents in an electronic facility.
The Corrosion of Electronic Components by Hydrogen Fluoride (HF) paper published in 2008 describes in detail the HF corrosion and provides the test results showing the corrosion damage due to HF. While HFC-227ea is a very good fire suppression system, the HFC-227ea thermal decomposition byproduct, Hydrogen Fluoride, would cause serious and expensive short and long term extensive corrosion damage by the condensed Hydrofluoric acid. Hydrogen Fluoride corrosion severely affects computers, servers, switching circuits, and other electronics, connectors and electromechanical devices.
To prevent corrosion caused by Hydrogen Fluoride, as well as other chemicals, acids, salts, and water which can be deposited during the activation of fire suppression systems or rapid humidity changes, all the electrical and electronic hardware, boards and connectors should be sprayed or coated with SuperCORR A, an ultra-thin film lubricant and corrosion preventive compound that is non-hardening and will not crack. SuperCORR A is a water displacing non-flammable compound that will not interfere with any type of electronics or avionics and prevents corrosion.
While a fire suppression system utilizing HFE-227ea will put out the fire, SuperCORR A will protect and prevent damage to the electronics or avionics caused by exposure to the Hydrogen Fluoride produced as a byproduct during the fire. SuperCORR A Mil-L-87177, is also effective in preventing damaging corrosion by atmospheric gases such as Sulfur dioxide (SO2), Nitrogen dioxide (NO2), Hydrogen Sulfide (H2S), Ammonia (NH3), and Chlorine based gases (CL2) in Class III mixed gas tests and fresh or salt water systems.
We can provide you with a Material Safety Data Sheets, independent laboratory reports, product samples and technical assistance.
For more information or advice please telephone us on +44 (0) 20 8281 6370 or use our contact form.
All products are supplied and supported by EnviroTech Europe Ltd. Manufactured in the United Kingdom and available on short delivery times through our dedicated team of distributors worldwide.
Share this page:

